Loving new things is the American way. The latest cars are better than old gas-guzzlers. New construction is safer than older construction. And in health care, cutting-edge technology or drugs are often considered better than what came before.
Attitudes about cancer care are no different. Adoption of technology—equipment, procedures or pharmaceuticals—for our industry tends to follow the technology adoption lifecycle theory, popularized by Everett Rogers in 1995 entitled Diffusion of Innovations1. Illustrated in this graph, the theory asserts that as groups of consumers (blue line) adopt new technology, its market share (yellow line) will eventually reach saturation.
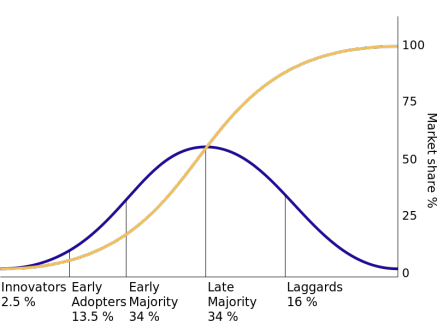
The Oncology Group has taken the general description of these groups’ profiles and applied them to cancer care.
- Innovators want something because it is new and when it is new. Often, there is no payment for innovative equipment in health care. They may be adopting new uses under clinical trials and are often found in academic settings.
- Early Adopters adopt innovation as a means to achieve a competitive advantage or benefit. They’ll be first-in-regional market for adopting technologies regardless of payment and long term results. Caution: this is the most risky time to buy if technology does not pan out. It is at this stage of the cycle that people pay the most for innovation.
- Early Majority (Pragmatists) adopt innovation when they see others adopt it. They invest in new technology when a volume of physicians consistently request it, and/or when peer-reviewed literature shows a clinical need and benefit.
- Late Majority (Conservatives) adopt innovation as a result of peer pressure but do so reluctantly. They invest in technology when losing patients or physicians because of their inability to attract patient volumes. When everyone else has a technology–and/or when data indicate the program is losing patient volume because of technology advances they haven’t yet acquired—then they’ll consider buying it.
- Laggards (Skeptics) never adopt innovation. Once a new technology becomes a proven standard one or once everyone else in the market has it, they embrace and purchase it.
But Is Anything Wrong With Tried and True?
Our industry is replete with stories of new technology that did not live up to expectations. Older technology may continue to be the standard. Some examples follow. The Oncology Group is reporting on this technology without commenting on it—we’re merely presenting examples of the old adage, "if it ain’t broke, don’t fix it."
Breast Cancer
Autologous Bone Marrow Transplant (ABMT) for Breast Cancer
ABMT for breast cancer was almost the standard of care in the late 1980s. Transplant centers were set up across the nation to care for women with breast cancer and women insisted—and sued for—their insurance to cover the procedure. During the late 1990s, numerous studies were published, some with conflicting information. By the first part of this century, the studies were conclusive: ABMT was no better than the alternative and had a much higher dollar and physical cost. Click here for an excellent discussion by Austin Frakt about the story of ABMT for breast cancer.
Accelerated Partial Breast Irradiation MammoSite
Partial Breast Irradiation has been performed for decades, in the past with the use of catheters in the operative site. The introduction of Accelerated Partial Breast Irradiation (APBI) applications such as MammoSite (Hologic) escalated the adoption.
This treatment was believed to revolutionize local breast cancer treatment, cutting treatment time from weeks (with external beam therapy) to five days. In early studies published in national medical journals (Archives of Surgery 2005 and American Journal of Surgery in 2007), five-year outcomes were equal to external beam and interstitial cavity brachytherapy.
Fast forward to this decade.
On January 2012, the NCI Bulletin reported on findings that APBI patients had a higher subsequent mastectomy rate than those women with whole breast radiation.
In September 2013, a study in The Journal of The American College of Surgeons from physicians at Dartmouth-Hitchcock Medical Center reported the results of a four-year follow-up study comparing women who had MammoSite for treatment vs. external beam. Those who were treated with MammoSite had significantly more palpable masses requiring three times more core biopsy to rule out malignancy and significantly more patients had telangiectasia (abnormal dilation of red, blue or purple superficial capillaries, arterioles or venules typically located just below the skin's surface) 2.
At the same time, at the American Society for Radiation Oncology 55th Annual Meeting, a 10-year study reported no significant difference between whole breast irradiation (WBI) and APBI in terms of disease-free survival, cosmetic results or contralateral breast failure.
All current studies (and ASTRO) do support using APBI for select patients. In 2009, ASTRO published a consensus statement.
The jury remains out on this one.
Treatment for Prostate Cancer
Among diseases, prostate cancer gets a lot of press, with technology for treatment often the focus. Watchful waiting hit the forefront more than a decade ago, but the public and physicians were resistant.
You have it in your medicine cabinet. You think nothing of it. It is cheap, easy to find and doesn’t require a prescription. No patent is in place. Hippocrates used its active ingredient to alleviate pain and fever.
For many years, studies have touted aspirin use for cardiac disease prevention—we now know to give a person experiencing a heart attack a baby aspirin. And studies are showing preliminary results supporting use of aspirin for cancer prevention. A British study found that a 75 mg dose daily taken for five years could reduce the risk of dying from common cancers by 21 percent. A Lancet study showed effectiveness in reducing the risk of adenocarcinomas. The NCI has a page devoted to Aspirin and "the promise in helping prevent cancer."
Technology Teams
Technology teams are utilized more frequently to determine the critical items needed for success. Oncology technology teams can review technology across the continuum, prioritizing them for purchase. Understanding where the program and the leadership/clinical team fall in the Innovation Adoption Lifecycle—and the team's appetite for risk-taking—may help you in planning for technology and selling it to those who manage the finances.
To learn more about the expertise of The Oncology Group and how we can help with technology purchases or team development, please contact Marsha Fountain, President of The Oncology Group, at 512.583.8815 or by email at [email protected].
1. (Rogers, E.M. (1995). Diffusion of innovations (4th edition). The Free Press. New York.) now part of the public domain.
2. Kari Rosencrantz, et al; Increased Rates of Long Term Complications after MammoSite Brachytherapy compared to Whole Breast Radiation Therapy. Journal of the American College of Surgeons. 2013: 217: 497-502
3. Jacobs BL, et al.Use of Advanced Treatment Technologies Among Men at Low Risk of Dying From Prostate Cancer. JAMA. 2013;309(24):2587-2595.
4. Julia H. Hayes, et al. Observation Versus Initial Treatment for Men With Localized, Low-Risk Prostate Cancer; A Cost-Effectiveness Analysis. Annals of Internal Medicine. 2013;158):853-860.
